Study Finds Single-Dose Cholera Vaccine as Effective as Standard Two-Dose Regimen in Cholera Endemic Setting
Among children under 5, protection might wane after third year
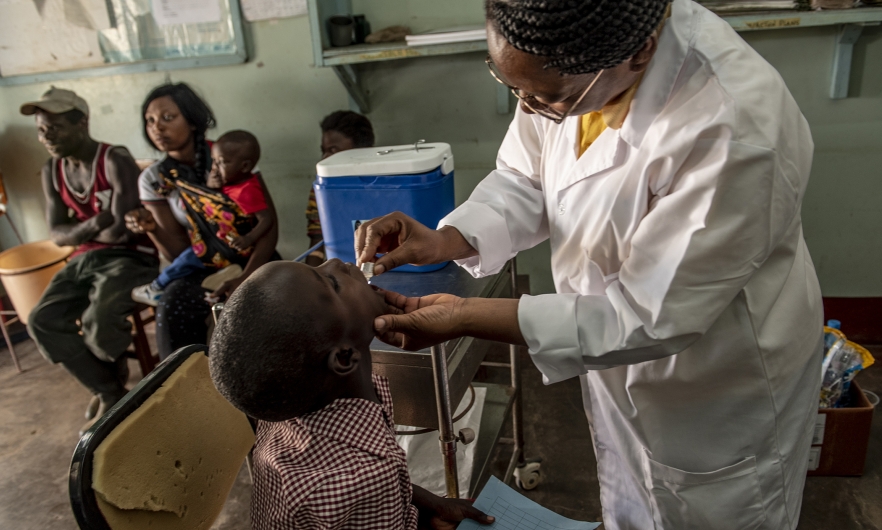
A health worker administers oral cholera vaccine to a child in Zambia. Photo credit: Karen Kasmauski
In the midst of a global cholera vaccine shortage and mounting cholera cases, a new study led by researchers at Johns Hopkins Bloomberg School of Public Health finds that a single dose of the vaccine provides significant protection against cholera for at least three years after being administered.
The study was published online January 18 in The Lancet Infectious Diseases.
Cholera is a bacterial disease that can cause severe diarrhea and dehydration and is typically spread through contaminated water and food. Worldwide there are as many as 4 million cases annually, according to the World Health Organization. The cholera vaccine is licensed as a two-dose regimen with with doses typically administered 14 days apart. Cholera vaccines are used in children and adults in both response to outbreaks and in endemic areas.
The demand for cholera vaccines in recent years has surpassed global supplies, leaving many populations without vaccine access. In response, the International Coordinating Group, which is based at the World Health Organization and manages the global emergency stockpile of vaccines, announced in October 2022 that it was temporarily halting the standard two-dose cholera vaccine regimen, replacing it with a single dose in cholera outbreak-response campaigns.
Evidence supporting single dose protection has been scant, particularly among children. For their study, the researchers turned to Uvira, an endemic urban setting in eastern Democratic Republic of the Congo where mass vaccination campaigns had been conducted in 2020 with the Euvichol-Plus vaccine, the only WHO-prequalified cholera vaccine widely available for use in cholera-affected communities.
For their study, the researchers recruited individuals with confirmed cholera from two vaccination sites across two distinct periods: October 14, 2021, to March 10, 2022 (12 to 17 months after vaccination) and November 21, 2022 to October 18, 2023 (24 to 36 months after vaccination). The researchers enrolled 658 individuals ages 1 to 86 with confirmed cholera cases, as well as 2,274 matched individuals for the control group.
The researchers found that adjusted single dose vaccine effectiveness was 52.7 percent 12 to 17 months after vaccination and 45.5 percent 24 to 36 months after vaccination.
“The results suggest that, at least in cholera endemic areas like Uvira, the use of one kOCV dose might provide significant protection for years rather than just months,” says Andrew Azman, PhD, Associate Scientist in the Bloomberg School’s Department of Epidemiology and a senior author of the paper.
While the evidence provides support for similar levels of protection in young children and others for up to 36 months, protection among children younger than 5 years might wane significantly during the third year after vaccination. The authors note that their estimate for vaccine effectiveness dropped considerably in the third year for young children. Because of the limited sample size of younger children, the authors cannot say with certainty how different protection is between young children and others in this time period. The authors add that other studies have shown lower protection in young children, so quicker waning would not be a surprise.
The authors note that more research is needed to assess a single-dose approach for those younger than 5 years, including the possibility of providing age-tailored dose schedules.
The study was supported by the Wellcome Trust and Gavi.