Discovery Illuminates How Sleeping Sickness Parasite Outsmarts Immune Response
Parasite spread by tsetse flies persists in hosts by hiding in tissues and evading antibodies
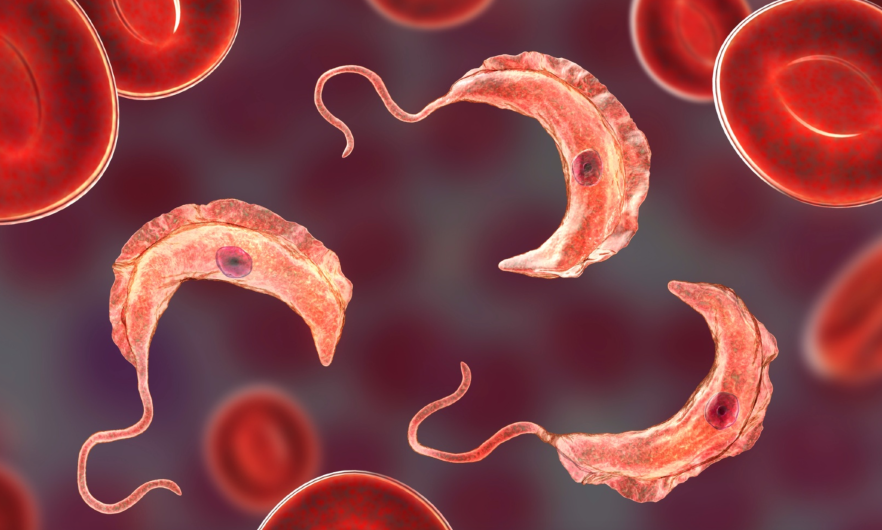
A new study led by researchers at the Johns Hopkins Bloomberg School of Public Health sheds light on how the blood-borne parasite that causes African sleeping sickness in humans and related diseases in cattle and other animals establishes long-term infections in hosts. Using a mouse model, the researchers showed thatTrypanosoma brucei essentially plays a game of hide-and-seek by setting up shop in its hosts’ tissues, allowing it to constantly change its protective surface coat and evade antibodies.
The discovery, reported October 30 in Nature, could potentially pave the way for understanding the immune response to other pathogens.
African sleeping sickness—also known as human African trypanosomiasis—is a neglected tropical disease that is usually fatal to humans if untreated. While treatment campaigns and tsetse fly control efforts have helped control human African trypanosomiasis, T. brucei remains a major problem for African farmers, killing an estimated three million cattle per year.
The T. brucei parasite constantly changes a surface coat made up of millions of copies of a single protein—the variant surface glycoprotein (VSG). Once one VSG has been recognized by a host’s antibody response, the parasite has already “switched” to a new one, which the immune system hasn’t spotted yet. By changing which of these variant genes is active, the parasite can alter its appearance enough to evade its host’s immune response for long periods.
The T. brucei parasite, after entering the bloodstream via a tsetse fly bite, also infects tissues outside the bloodstream. The function of this “extravascular” home for the parasite had previously been unclear. To get a fuller understanding of how T. brucei evades the immune system, the researchers used a customized RNA sequencing method to catalog the distinct VSG types that appeared over time during T. brucei infections in mice.
The researchers discovered that the vast majority of VSGs arose in tissues rather than in the bloodstream. They also found that parasite clearance by the immune system is slower in tissues. Their findings suggest that moving from the bloodstream into extravascular spaces gives T. brucei important “breathing room” in which to generate enough VSG variants to survive long-term in hosts, ensuring continued transmission.
“This work sets the stage for a new way of thinking about immune evasion in T. brucei infection and potentially other chronic infections,” says study senior author Monica Mugnier, PhD, associate professor in the Bloomberg School’s Department of Molecular Microbiology and Immunology.
The study’s first author, Alexander Beaver, PhD, was a doctoral candidate in the Mugnier laboratory at the time of the study.
Transmitted by the tsetse fly, T. brucei parasites are found in many parts of sub-Saharan Africa. Many wild mammals in Africa harbor the parasite without showing signs of disease, and thus serve as reservoirs. In humans, and in livestock such as cattle, sheep, and goats, T. brucei infection can cause a chronic disease that, in later stages, involves severe lethargy and other neurological symptoms.
The parasite enters its mammalian hosts via the bloodstream during a tsetse fly bite, and usually exits the same way. Why T. brucei spreads from the blood into extravascular spaces has been an unresolved question. In the study, Mugnier and her team used their own VSG-targeted RNA-sequencing method to record the expression of different VSGs over time in T. brucei parasites recovered from both the blood and tissues of infected mice.
They showed that once an infection had been established for more than a week or so, the number of detectable VSGs in mouse tissues was, on average, several times higher than the number in blood. Thus, the bulk of VSG diversity—the key determinant of the pathogen’s ability to evade the immune response—was seen in tissues, not blood. This was the case whether the mice were infected by needle or by tsetse fly bite.
When the researchers tracked specific VSGs from initial infection, they found that immune clearance of parasites bearing these VSGs occurred significantly later in tissues than in blood. Similarly, when they used mice engineered so that tissue clearance of the parasite was further delayed, tissue VSG diversity was correspondingly greater.
The results overall suggest that T. brucei uses tissues as relatively protected spaces in which it can survive longer, generating a greater diversity of variants, and potentially re-seeding the bloodstream with these variants—faster than the immune system can keep up.
“Tissues were once thought to be incidental sites of infection for T. brucei, but it now appears that they might play an important role in maintaining a long-term infection for this parasite, while the bloodstream may serve more as a highway system for movement between tissues and for eventual transmission back into the tsetse fly,” Mugnier says.
This new model suggests in turn, she adds, that disrupting T. brucei’s ability to spread from blood to tissues could be enough to allow the immune system to catch up and terminate infection, and thus could be a valuable new treatment strategy.
Mugnier and colleagues also suspect that this exploitation of extravascular spaces may be a tactic used by other pathogens—the Lyme disease bacterium, for example—to establish chronic infections.
“T. brucei infection could turn out to be a useful model for understanding why extravascular spaces are less efficient at pathogen clearance and how some pathogens exploit that,” Mugnier says.
“Tissue spaces are reservoirs of antigenic diversity for Trypanosoma brucei” was co-authored by Alexander Beaver, Zhibek Keneskhanova, Raul Cosentino, Brian Weiss, Erick Awuoche, Gretchen Smallenberger, Gracyn Buenconsejo, Nathan Crilly, Jaclyn Smith, Jill M.C. Hakim, Bailin Zhang, Bryce Bobb, Filipa Rijo-Ferreira, Luisa Figueiredo, Serap Aksoy, T. Nicolai Siegel, and Monica Mugnier.
Support for the study was provided by the National Institutes of Health (T32AI007417, T32OD011089, 1K99GM132557-01, DP5OD023065, R01AI58805), the Chan Zuckerberg Initiative, the German Research Foundation and the European Research Council.
# # #
Media contacts: Kathy Marmon kmarmon@jhu.edu or Kris Henry khenry39@jhu.edu